Novavax Initiates Phase 2 Safety and Immunogenicity Trial to Evaluate Annual Re-Dosing of the RSV F Vaccine in Older Adults
GAITHERSBURG, Md., Oct. 22, 2015 (GLOBE NEWSWIRE) -- Novavax, Inc. (Nasdaq:NVAX), a clinical-stage vaccine company focused on the discovery, development and commercialization of recombinant nanoparticle vaccines and adjuvants, today announced that enrollment has begun in a Phase 2 rollover clinical trial of its respiratory syncytial virus F-protein nanoparticle vaccine candidate (RSV F Vaccine) in older adults enrolled in the prior Phase 2 trial.
The trial is a randomized, observer-blinded, placebo-controlled rollover trial designed to enroll the same 1600 adults 60 years of age and older (older adults) who participated in the recently concluded prior Phase 2 trial. Participants previously randomized to receive 135µg RSV F Vaccine or placebo will be re-enrolled and re-randomized in the current trial to receive either 135µg RSV F Vaccine or placebo. This will result in analysis of four separate study arms: a) participants receiving RSV F Vaccine in both the first trial and second trial; b) participants receiving placebo in the first trial and RSV F Vaccine in the second trial; c) participants receiving RSV F Vaccine in the first trial and placebo in the second trial; and d) participants receiving a placebo in both the first trial and second trial.
The primary endpoints of the trial will evaluate safety and serum anti-F IgG antibody concentrations in response to immunization with the RSV F Vaccine. Secondary endpoints will examine palivizumab-competing antibody (PCA) concentration and neutralizing antibody titer to at least one RSV/A and one RSV/B strain.
"It is estimated that 2.4 million adults 65 years of age or older are infected with RSV annually in the U.S., leading to as many as 900,000 medical interventions and 14,000 deaths each year," said Stanley C. Erck, President and CEO. "This RSV F Vaccine rollover trial will provide important information on the amplitude and duration of immunogenicity in older adults, which will be a key data set as we develop the RSV F Vaccine for annual, seasonal vaccination."
About Novavax
Novavax, Inc. (Nasdaq:NVAX) is a clinical-stage vaccine company committed to delivering novel products to prevent a broad range of infectious diseases. Our recombinant nanoparticles and Matrix-M(TM) adjuvant technology are the foundation for groundbreaking innovation that improves global health through safe and effective vaccines. Additional information about Novavax is available on the Company's website, novavax.com.
Forward-Looking Statements
Statements herein relating to the future of Novavax and the ongoing development of its vaccine and adjuvant products are forward-looking statements. Novavax cautions that these forward looking statements are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those expressed or implied by such statements. These risks and uncertainties include those identified under the heading "Risk Factors" in the Novavax Annual Report on Form 10-K for the year ended December 31, 2014, filed with the Securities and Exchange Commission (SEC). We caution investors not to place considerable reliance on the forward-looking statements contained in this press release. You are encouraged to read our filings with the SEC, available at sec.gov, for a discussion of these and other risks and uncertainties. The forward-looking statements in this press release speak only as of the date of this document, and we undertake no obligation to update or revise any of the statements. Our business is subject to substantial risks and uncertainties, including those referenced above. Investors, potential investors, and others should give careful consideration to these risks and uncertainties.
Contact: Novavax, Inc. Barclay A. Phillips SVP, Chief Financial Officer and Treasurer Andrea N. Flynn, Ph.D. Senior Manager, Investor Relations ir@novavax.com 240-268-2000 Russo Partners, LLC David Schull david.schull@russopartnersllc.com Todd Davenport, Ph.D. todd.davenport@russopartnersllc.com 212-845-4271
The issuer of this announcement warrants that they are solely responsible for the content, accuracy and originality of the information contained therein.
Source: Novavax, Inc. via Globenewswire
ARIVA.DE Börsen-Geflüster
Aktie im Fokus
|
Atha Energy Corp
0,43
€
-2,71% 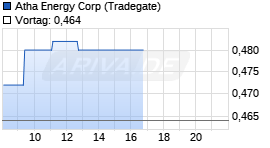 |
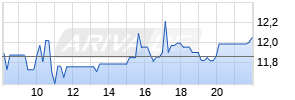
Kurse
 |
Mehr Nachrichten zur Novavax Aktie kostenlos abonnieren
(Mit der Bestellung akzeptierst du die Datenschutzhinweise)

Hinweis: ARIVA.DE veröffentlicht in dieser Rubrik Analysen, Kolumnen und Nachrichten aus verschiedenen Quellen. Die ARIVA.DE AG ist nicht verantwortlich für Inhalte, die erkennbar von Dritten in den „News“-Bereich dieser Webseite eingestellt worden sind, und macht sich diese nicht zu Eigen. Diese Inhalte sind insbesondere durch eine entsprechende „von“-Kennzeichnung unterhalb der Artikelüberschrift und/oder durch den Link „Um den vollständigen Artikel zu lesen, klicken Sie bitte hier.“ erkennbar; verantwortlich für diese Inhalte ist allein der genannte Dritte.